Adverse Effects Implementation Guide
What are adverse effects?
Adverse effects are unintended pharmacological effects that occur when a drug product is administered correctly. Knowing the adverse effects of a drug is important because not only do we want to ensure patients are benefiting from the drug, we want to ensure any associated health risks are minimal.
How Does the Adverse Effects Search Work?
DrugBank's Adverse Effects data is well-structured and contains information about drug adverse effect profiles such as incidence, evidence type, patient characteristics, and other details related to the adverse effect. There is more than one way to connect to DrugBank's Adverse Effects endpoints. Here we will focus on how to call the API by searching for a particular adverse effect.
This implementation method is well-suited for looking up a list of drugs for which a specific adverse effect has been reported.
NOTE: When using the Adverse Effect endpoint, you are searching for a condition which will return adverse effects related to those conditions. At DrugBank, a condition id starts with the letters DBCON, followed by 7 numerical digits (eg. DBCOND0000001)
Step 1
To start an adverse effects search, you would enter the following:
https://api.drugbank.com/v1/adverse_effects?q=Copy to clipboard
Step 2
Enter in the adverse effect you want to look up e.g. nausea
https://api.drugbank.com/v1/adverse_effects?q=nauseaCopy to clipboard
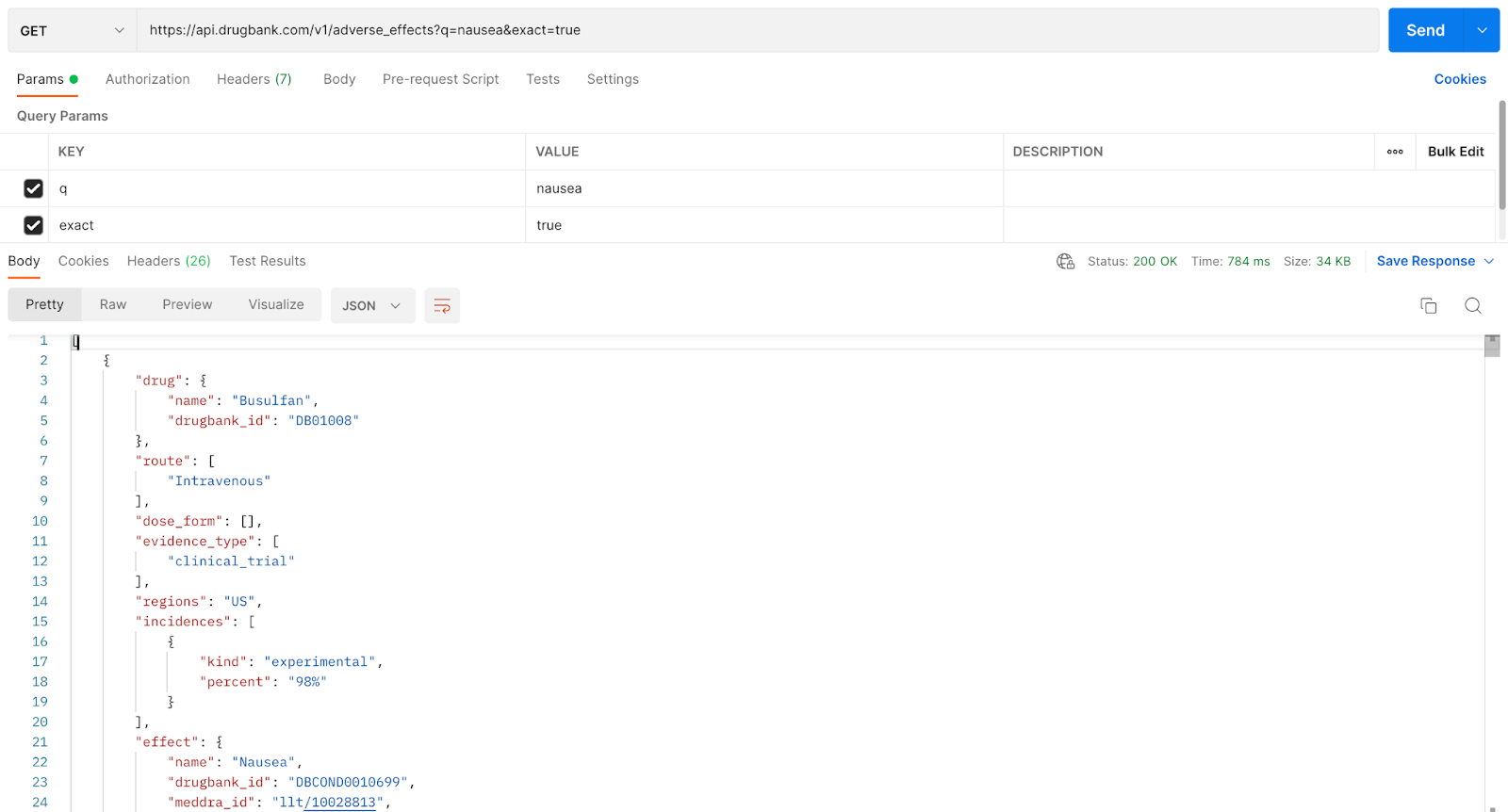
NOTE: Let's take a look at the first hit that's returned. You can see that there is also a parameter set where exact=true. By setting this parameter, partial matches such as 'nausea and vomiting' are not returned; only the queried text 'nausea' was matched.
This response reads, “The drug Busulfan (line 4) was administered intravenously (line 8) in a clinical trial (line 12) that was carried out in the US (line 14). 98% of the patients (line 18) who took the experimental drug (line 17) reported an adverse effect of nausea (line 22).
Another point to mention is the Adverse Effects endpoint may include or exclude “patient characteristics.” In this first response, a patient with prior allogeneic hematopoietic cell transplantation (line 30), which is a procedure that involves a patient receiving a donor's bone marrow or blood may feel nauseated when given Busulfan. Providing the patient's characteristic gives additional context to the adverse effect in question, which may help researchers, and the like, make better-informed decisions.
TIP: We can see that the adverse effect “nausea” is connected to the drugbank_id: DBCON0010699 in line 23. External IDs such as MedDRA, SNOMED and ICD-10 are also mapped in lines 24, 25, and 26, respectively. This is beneficial for customers who already have an existing system with these external IDs, which will allow for easier integration.
Important fields for Adverse Effects
The Adverse Effects data through DrugBank's API contains several pieces of information, though some of those returned may not be as beneficial depending on a user's needs. For instance, the evidence_type array may have results originating from clinical trials, uncontrolled trials, varying reports, etc., and the incidences array will have a “kind” field such as experimental and comparator and a “percent” value connected to the “kind” specified. If only certain criteria of these arrays are needed, you may filter out certain responses so as not to overwhelm the list returned in the call. For instance, in your application, incidence percent values of <2% may not be a significant percentage reported for an adverse effect, and you may choose to filter out returns where the hits produced are >2%.
Here is some common information from the Adverse Effects search that may be of interest:
| Fields | Description |
|---|---|
| evidence_type | This will display where the reported adverse effect originated from |
| region | The jurisdiction that the adverse effect was reported from |
| Incidences→ kind, percent | Kind: specify the type of the drug that was administered whether it was the experimental drug, a placebo, a
comparator drug Percent: shows the percent of patients who've reported the adverse effect based on the incidence kind that was received |
| patient_characteristics | This provides additional information of the patients who have reported the adverse effect. Things such as the age of the patient, underlying medication conditions, additional therapies and treatments that the patient may be going through. |
Good To Know
Adverse effects can be mapped to a list of related drugs by adding /drugs to the end of the call:
https://api.drugbank.com/v1/adverse_effects/drugsCopy to clipboard
Additional information such as references for the drugs can be obtained by setting this parameter:
| Parameter | Default | Description |
|---|---|---|
| include_references | false | If |
Adverse effects can be mapped to a list of related drug products by adding “/products” to the end of the call:
https://api.drugbank.com/v1/adverse_effects//productsCopy to clipboard
Additional information such as the ingredient descriptions for the drug products can be obtained by using these parameters:
| Parameter | Default | Description |
|---|---|---|
| include_simple_desc | false | If set to |
| include_clinical_desc | false | If set to |