Adverse Effects via Medication Search Implementation Guide
What are Adverse Effects?
Adverse effects are unintended pharmacological effects that occur when a drug product is administered correctly. Knowing the adverse effects of a drug is important because not only do we want to ensure patients are benefiting from the drug, we want to ensure any associated health risks are minimized.
How Does the Adverse Effects via the Medication Search Work?
DrugBank's Adverse Effects data is well structured and contains information about drug adverse effect profiles such as incidence, evidence type, patient characteristics and other details related to the adverse effect. There is more than one way to connect to DrugBank's Adverse Effects endpoints. Here we will focus on showing you how to obtain a list of adverse effects reported for a specific drug product.
Note: When using the Adverse Effect endpoint, it is connected to product concepts via conditions, so part of the search involves searching a particular condition which returns it as an adverse effect. At DrugBank, a condition ID starts with the letters DBCON, followed by 7 numerical digits (e.g. DBCOND0000001)
Let's take a look at this scenario:
A Physician is interested in starting treatment of busulfan in a patient diagnosed with chronic myelogenous eukemia. The patient also has underlying heart problems, and the physician plans to add busulfan into the patient's medication regimen but is unaware of the adverse effects of the drug.
He can utilize the Medication Search endpoint using the product concept ID to verify the adverse effects of busulfan. There are several reported adverse effects (such as Nausea, Vomiting, Stomatitis, Hypomagnesemia, etc…) from the use of busulfan. He sees that one of the adverse effects is tachycardia, which is a heart rate that's too fast. The physician can then note that busulfan as treatment for chronic myelogenous leukemia should not be recommended for this patient, and alternative treatments should be searched instead.
Step 1
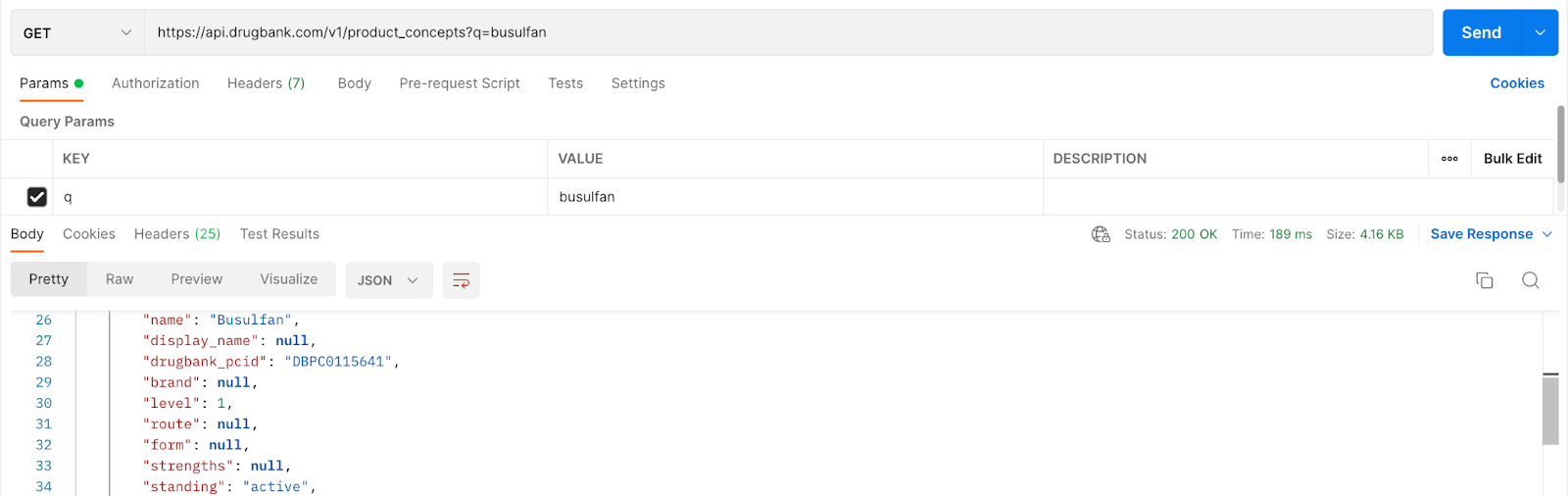
Using the base URL for the medication search, obtain the product concept id for busulfan:
Step 2
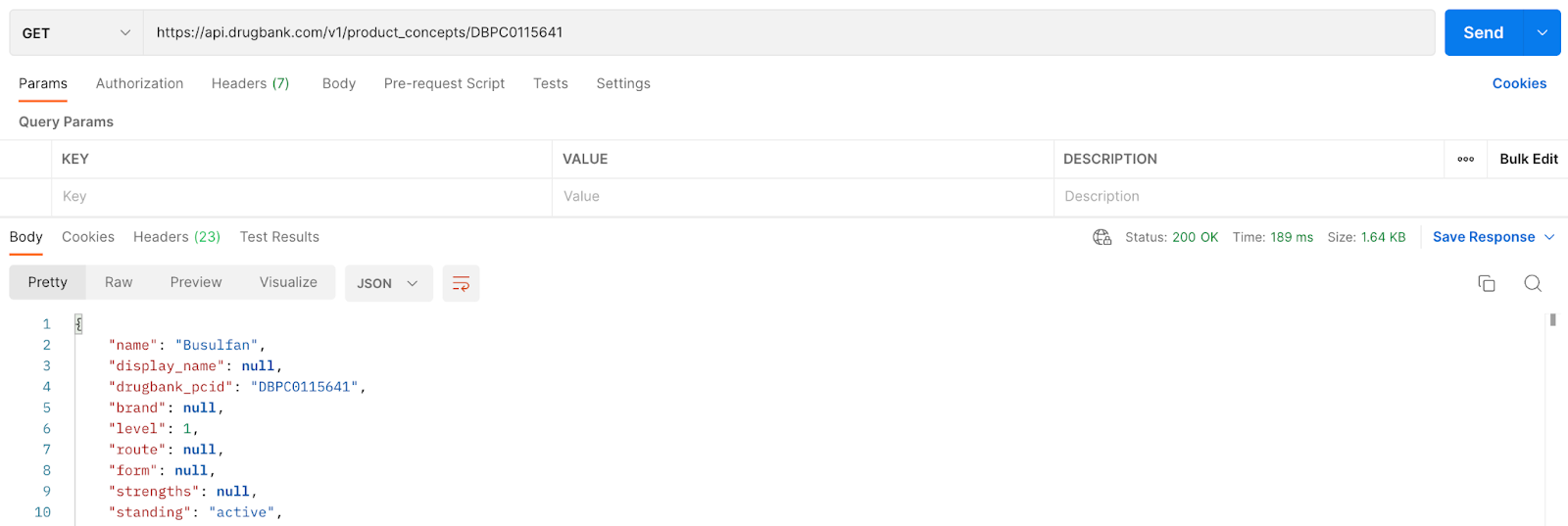
Grab and store the drugbank_pcid: DBPC0115641
Step 3
To get the adverse effects related to the Product Concept ID saved in Step 2. Add “adverse_effects” to the end of the call:
https://api.drugbank.com/v1/product_concepts/DBPC0014482/adverse_effectsCopy to clipboard
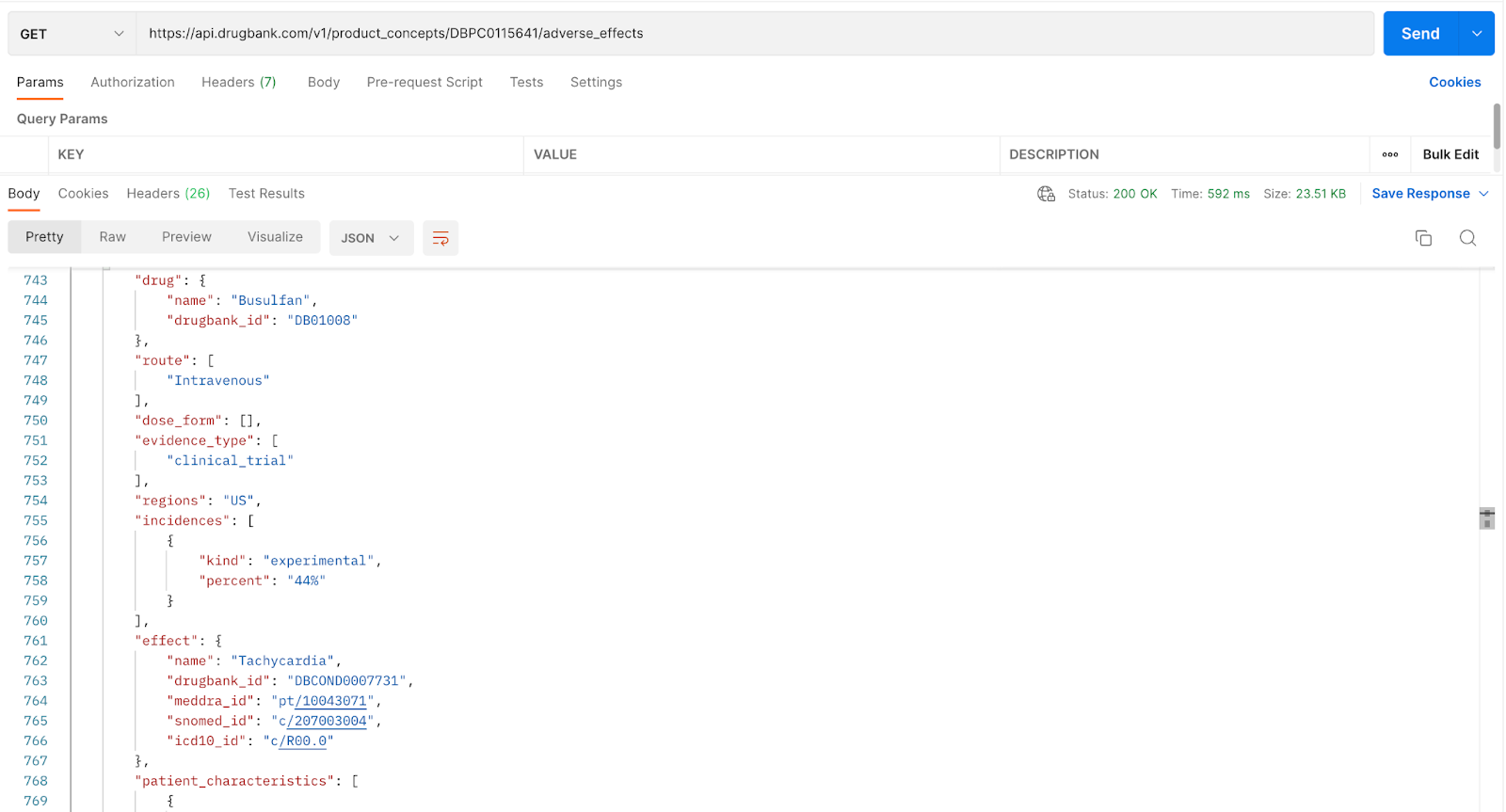
Note: The results of this call produced numerous hits for the adverse effects of busulfan. Here we're showing a snippet of the return of interest, which reads, “The drug Busulfan (line 744) was administered intravenously (line 748) in a clinical trial (line 752) that was carried out in the US (line 754). 44% of the patients (line 758) who took the experimental drug (line 577) reported an adverse effect of Tachycardia (line 762).
Another point to mention is the adverse effects may include or exclude “patient characteristics,” which provides additional context to the adverse effect in question. In this response, a patient with prior allogeneic hematopoietic cell transplantation (line 770), which is a procedure that involves a patient receiving a donor's bone marrow or blood, may experience tachycardia when given Busulfan. Providing the patient's characteristic gives additional context to the adverse effect in question, which may help researchers and the like make better-informed decisions.
Tip: We can see that the adverse effect tachycardia is connected to the condition assigned with “drugbank_id”: “DBCOND0007731” in line 763. External IDs such as MedDRA, SNOMED and ICD-10 are also mapped in lines 764, 765, 766, respectively. This is beneficial for customers who already have an existing system with these external IDs, which will allow for easier integration.
Important fields for Adverse Effects
The Adverse Effects data through DrugBank's API contains several pieces of information, though some of those returned may not be as beneficial, depending on your needs. For instance, the evidence_type array may have results originating from clinical trials, uncontrolled trials, varying reports, etc… and the incidences array will have a “kind” field such as experimental, placebo, comparator, and a “percent” value connected to the “kind” specified. If only certain criteria of these arrays are needed, you may filter out certain responses so as not to overwhelm the list returned in the call. For instance, if you are only interested in returns where the reported adverse effects are from clinical trial patients who were administered the experimental drug, you could filter results to show only when evidence_type: clinical trial and the kind: experimental.
Here is some common information from the adverse effects search that may be of interest:
| Fields | Description |
|---|---|
| evidence_type | This will display where the reported adverse effect originated from |
| region | The jurisdiction that the adverse effect was reported from |
| Incidences→ kind, percent | Kind: specify the type of the drug that was administered whether it was the experimental drug, a placebo, a comparator drug Percent: shows the percent of patients who've reported the adverse effect based on the incidence kind that was received |
| patient_characteristics | This provides additional information of the patients who have reported the adverse effect. Things such as the age of the patient, underlying medication conditions, additional therapies and treatments that the patient may be going through. |
Additional Helpful Parameters
No matter your use case, our Clinical API provides a range of powerful tools to help you bring more certainty and speed to you and your user's day. Depending on your specific needs, it's a good idea to explore a variety of DrugBank's options to find the right module for you. Our customer success team has the know-how and experience to help you identify what will work best for you and guide you along the way as you get up and running.
| Parameter | Default | Description |
|---|---|---|
| include_references | false | If |